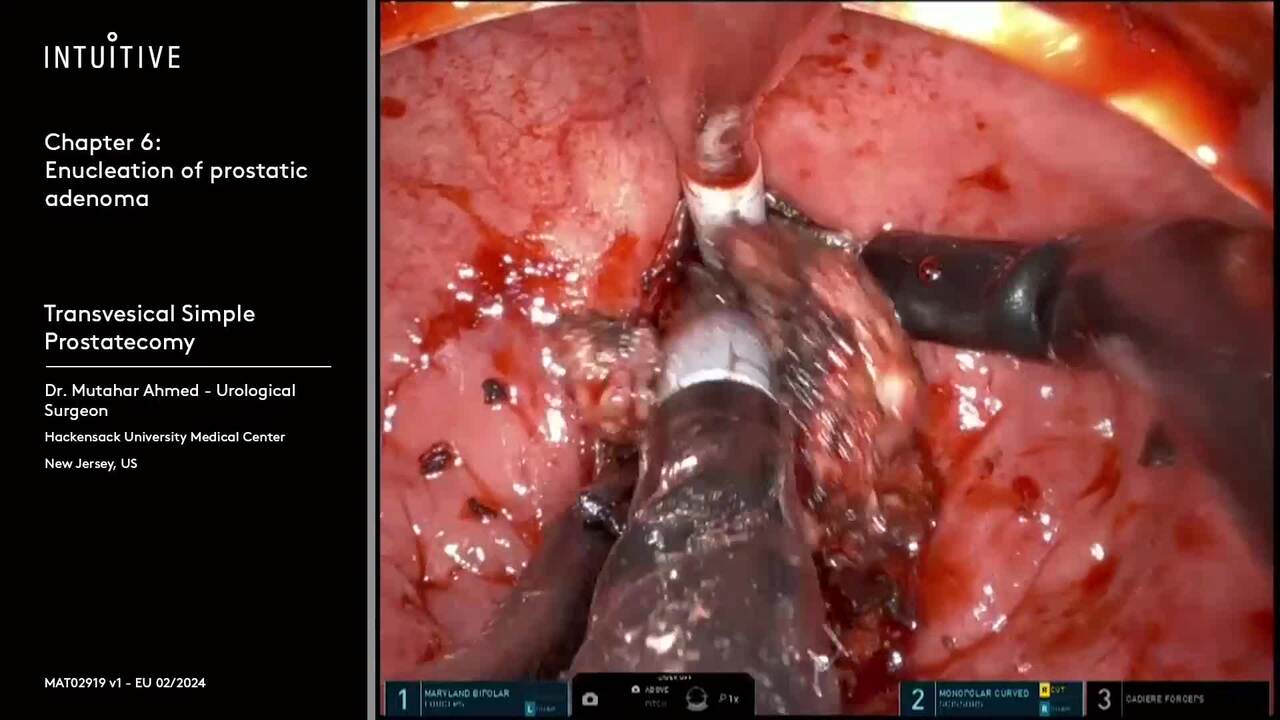
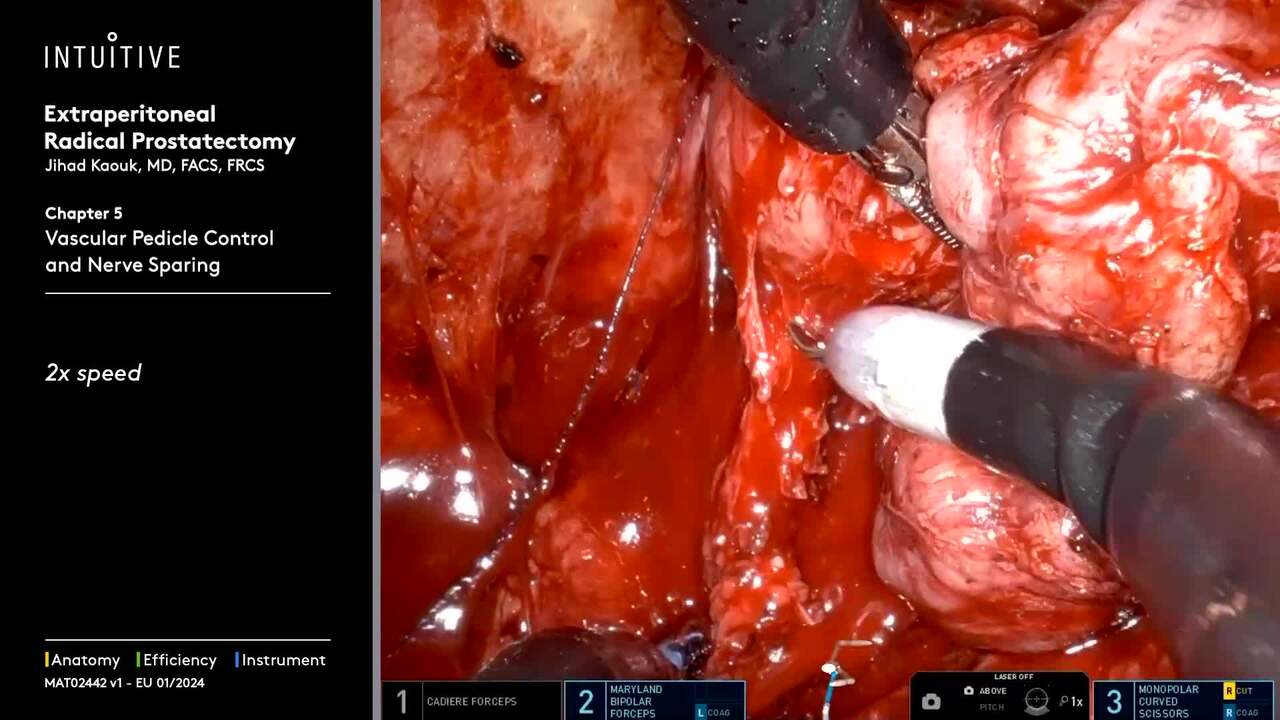
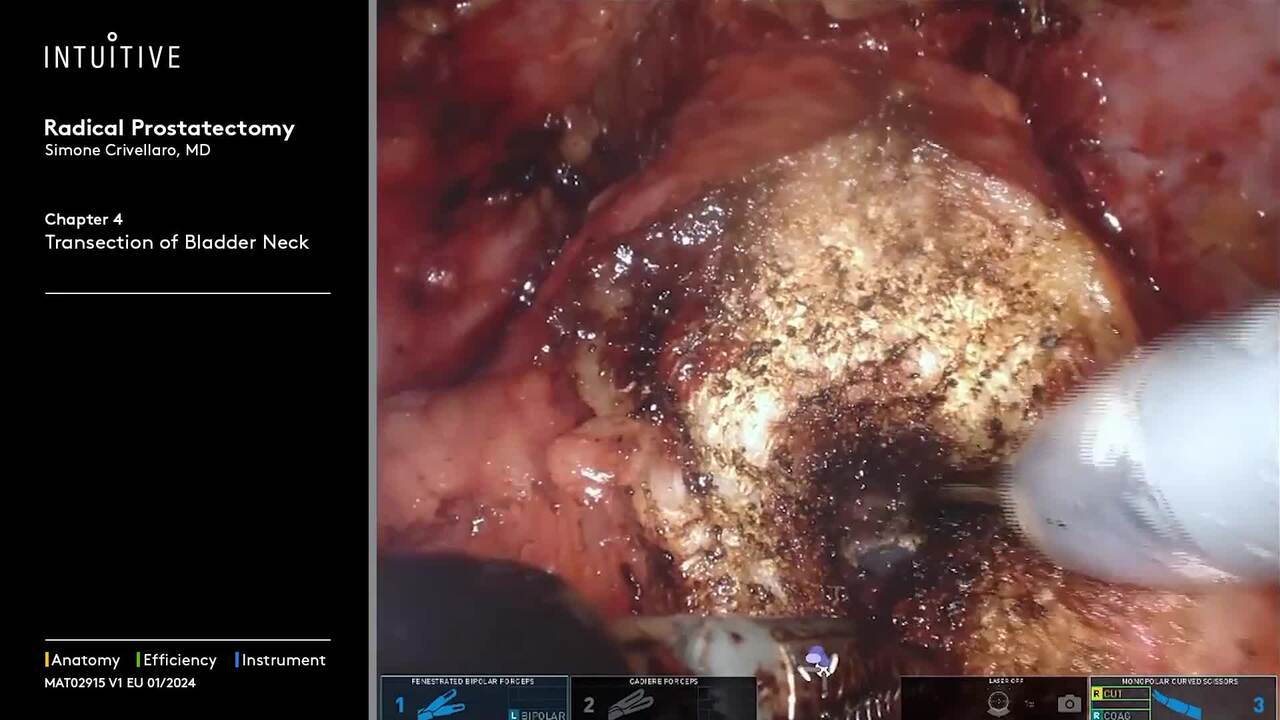
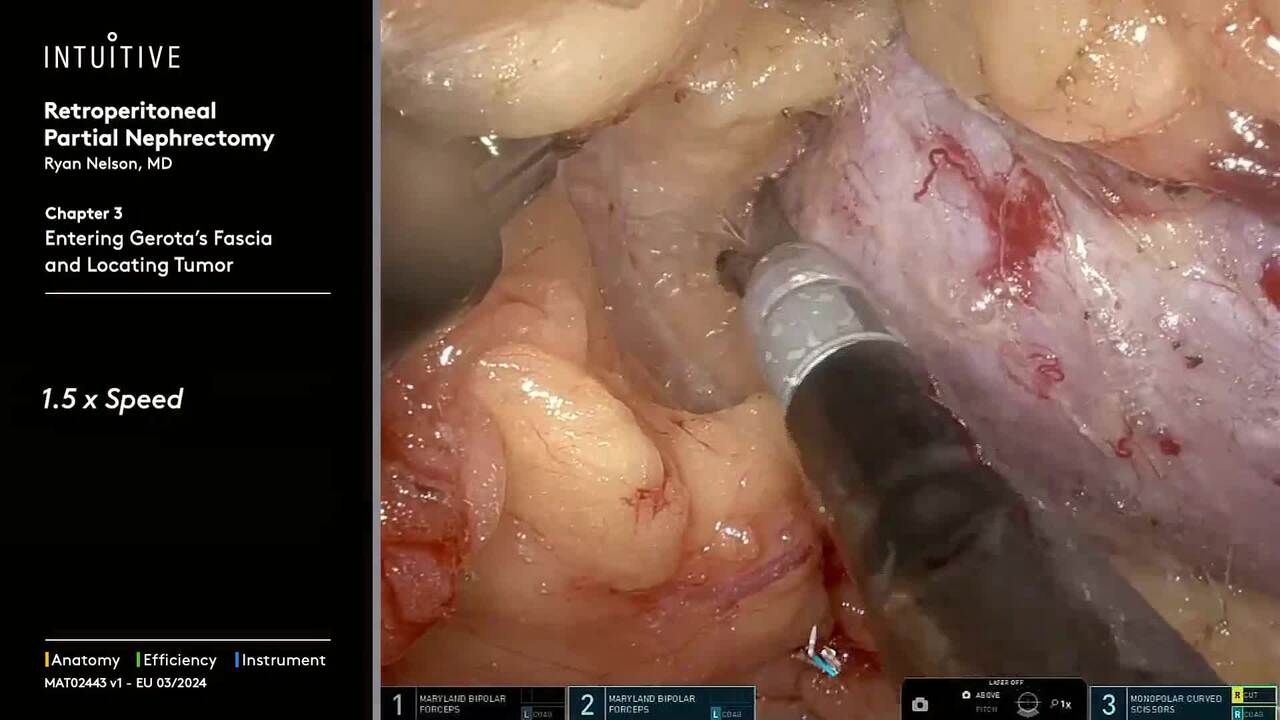
Perform procedures with a range of complexity using da Vinci SP, which allows access through a single port—enabling surgical innovation for procedures like extraperitoneal prostatectomy and retroperitoneal partial nephrectomy.
For urologic surgeons, the da Vinci Xi system offers multi-port robotic-assisted surgery. Multi-quadrant access allows surgeons to perform a variety of procedures by offering broad anatomical access and integration with advanced technology.
With My Intuitive App, access your da Vinci procedure data to gain insights about your practice or program. Continuously evolve by tracking metrics and identifying opportunities for growth.
Staple with complete control, full articulation, and intelligent feedback. At SureForm’s core is SmartFire technology which monitors tissue compression before and during firing. We offer a comprehensive portfolio of staplers and reloads to ensure you have the right instrument to meet your procedural needs.