
Helping reduce the time from identification to intervention

Cancer is relentless, taking more lives globally than any other disease.1 Even among the more prevalent types, like lung cancer, approximately 72% of patients with lung cancer are diagnosed with non-localized disease,2 potentially limiting intervention options and reducing 5-year survival rates.
For patients diagnosed with cancer, time matters. A delay in any part of the patient pathway can result in a direct impact on prognosis. By aiming to bridge the gap between diagnosis and intervention, we believe we can help care teams shorten the patient journey.
The pathway for patients with suspicion of lung cancer can begin in different ways. Today, fewer than 7% of eligible high-risk patients in the U.S. get screened with a low dose CT scan,3 while other suspicious lesions (growths) are often found through an incidental finding.
If a suspicious lesion is detected, patients are triaged for potential biopsy. A recent study found that 46% of patients require two or more biopsies to receive a diagnosis with an average time of 57 days between the first biopsy and diagnosis.4 If diagnosis is not possible, it may be suggested that patients enter watchful waiting, where they would receive regularly scheduled imaging to assess if the lesion has grown. Patients may also be scheduled for surgery or oncologic therapy out of caution if there is a high risk of malignancy.
For the patients where a diagnosis comes from a biopsy, they may be referred for surgery, oncology services, or both, depending on the state of the disease. Each of these steps can take time and may result in delays, which may have a direct impact on prognosis.5 The variability at each stage of care coordination highlights an opportunity to examine and innovate for unmet needs on the lung care continuum.
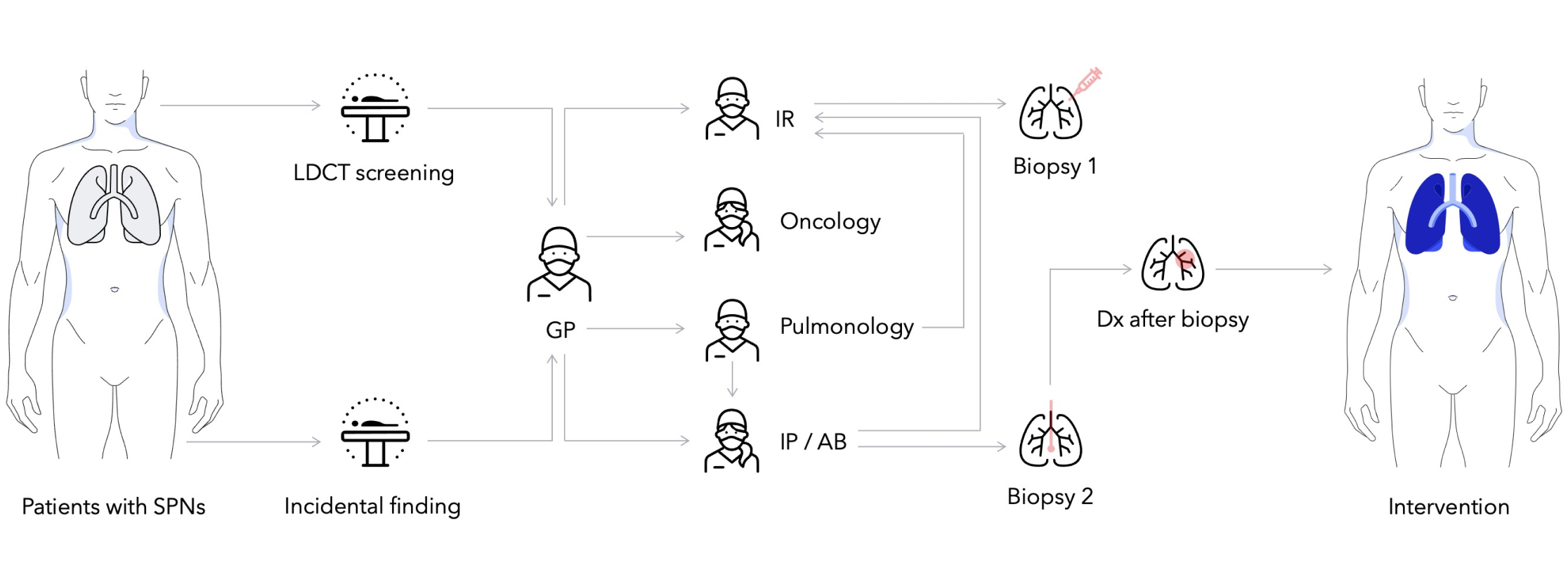
With approximately 70% of all-stage cancerous lung nodules found in the periphery of the lung,6 physicians may face challenges using bronchoscopy to biopsy peripheral nodules, including those less than 2 cm.7 Of note, small nodules less than 2 cm may represent early-stage disease (Stage IA1- IA2)—and detection of early-stage disease is associated with the highest 5-year survival rate of 92% and 83% respectively.8
Biopsy in the periphery may be done percutaneously and while chances of diagnosis are favorable, risks of complications are relatively high, including the risk of pneumothorax requiring chest tube of up to 15%9. With both approaches, multiple biopsies may be required to get a definitive diagnosis, which can add up to 90 days4 to a patient’s diagnostic journey, and up to an 80% increase in hospital cost.10
The introduction of Ion’s technology opens the door to other pathways of care. With Ion, physicians can navigate to and biopsy small nodules in the periphery10 to help enable quick care decisions. Unlike other navigation bronchoscopy systems, Ion’s fiber optic shape sensor is immune to electromagnetic interference, meaning it can be used in the procedure room alongside metallic items because it is not sensitive to metal objects.11
Considering that robotic-assisted surgery is currently the primary surgical modality used for lung resection12, biopsy enabled by Ion allows for pathology, staging, and surgical intervention using the da Vinci surgical system, to be performed sequentially in a single operating room.
Intuitive’s advanced teams in human factors, data, and research and development actively collaborate with hospitals across the U.S. to understand and help streamline workflows and support innovations that can shorten the critical time between detection and intervention.
Some hospitals are piloting an approach for appropriate patients that includes Ion’s lung nodule biopsy with 3D lung modeling,13 and surgical intervention into a single-anesthesia, multi-procedure case, completed in a single day, and in some cases, in a single operating room.
“Driven by the high stakes of this challenge, leading cancer centers are working with our teams in software and robotics, integrating systems and data to create new workflows that can help their care teams deliver diagnoses and minimally-invasive interventions more quickly, and at scale,” said Gary Guthart, CEO of Intuitive.

Addressing the gap between diagnosis and intervention represents a promising opportunity to continue the fight against lung cancer, as well as other diseases. Intuitive teams work closely with clinicians and hospitals to identify areas of care that could leverage similar or new applications to continue making a difference for its customers, for healthcare, and especially for patients.
Learn how one hospital is using the Ion system to help them drive change in lung cancer detection. Complete the form below to receive an email with the case study.
Thank you for your submission.
Ion is for sale in the U.S.
Outside of the U.S., Ion is not CE Marked and not for human use. Ion cannot be placed on the market or put into service. Ion may not have regulatory approvals in all markets. Please check with your local Intuitive representative.
Important safety information
Risks associated with bronchoscopy through an endotracheal tube and under general anesthesia are infrequent and typically minor, and may include but are not limited to: sore throat, hoarseness, respiratory complications including dyspnea or hypoxemia, airway injury, bronchospasm, laryngospasm, fever, hemoptysis, chest or lung infection including pneumonia, lung abscess or an adverse reaction to anesthesia. Although rare, the following complications may also occur: bleeding, pneumothorax (collapsed lung), cardiac related complications, respiratory failure, air embolism, or death. As with other medical procedures, there may be additional risks associated with the use of general anesthesia and/or endotracheal intubation which are not listed above; you should consult a health care professional regarding these and other potential risks.
Procedures using the Ion Endoluminal System may be associated with longer procedure and/or longer anesthesia time.
Patients should talk to their doctor to decide if da Vinci surgery is right for them. Patients and doctors should review all available information on non-surgical and surgical options and associated risks in order to make an informed decision.
Serious complications may occur in any surgery, including da Vinci surgery, up to and including death. Serious risks include, but are not limited to, injury to tissues and organs and conversion to other surgical techniques which could result in a longer operative time and/or increased complications. For Important Safety Information, including surgical risks, indications, and considerations and contraindications for use, please also refer to www.intuitive.com/safety.
Individuals' outcomes may depend on a number of factors, including but not limited to patient characteristics, disease characteristics and/or surgeon experience.
Ion endoluminal system
The Ion Endoluminal System (Model IF1000) assists the user in navigating a catheter and endoscopic tools in the pulmonary tract using endoscopic visualization of the tracheobronchial tree for diagnostic and therapeutic procedures. The Ion Endoluminal System enables fiducial marker placement. It does not make a diagnosis and is not for pediatric use.
The Flexision Biopsy Needle is used with the Ion Endoluminal System to biopsy tissue from a target area in the lung.
The PlanPoint Software uses patient CT scans to create a 3D plan of the lung and navigation pathways for use with the Ion Endoluminal System.